sas A00-280 Exam Questions
Questions for the A00-280 were updated on : Feb 20 ,2026
Page 1 out of 7. Viewing questions 1-15 out of 99
Question 1
Given the following SAS program:
Which statement correctly identifies invalid values in the variable TRT, if only the values 'A', B', 'C are
valid?
- A. if indexc(TRT, 'ABC') eq 0 then output;
- B. if index(TRT, 'ABC') eq 0 then output;
- C. if find(TRT, 'ABC') eq 0 then output;
- D. if indexw(TRT, 'ABC') eq 0 then output;
Answer:
A
Question 2
A Statistical Analysis Plan describes a clinical trial as "A 12 week, double-blind, placebo-controlled,
randomized, multi-center study." Double-blind refers to which groups in this study?
- A. treatment and control group
- B. investigator and subjects
- C. statistician and sponsor
- D. sponsor and investigator
Answer:
B
Question 3
A patient received at least one dose of study medication prior to withdrawing from a study. Which
analysis population would always include this patient?
- A. efficacy
- B. intent to treat
- C. per protocol
- D. safety
Answer:
D
Question 4
What is an international ethical and scientific quality standard for designing, conducting, recording
and reporting trials that involve the participation of human subjects?
- A. 21 CFR Part 11
- B. Good Clinical Practices
- C. MedDRA
- D. WHODrug
Answer:
B
Question 5
What is the main focus of 21 CFR Part 11?
- A. electronic submission requirements
- B. trial safety requirements
- C. statistical calculation requirements
- D. trial protocol requirements
Answer:
A
Question 6
An action plan that describes what will be done in a drug study, how it will be conducted, and why
each part of the study is necessary is called:
- A. a clinical trial plan
- B. a protocol
- C. a data management plan
- D. a statistical analysis plan
Answer:
B
Question 7
From the Statistical Analysis Plan, patients age is calculated as an integer relative to date randomized
divided by 365.25. Given the following annotated CRF:
Which programming code defines the patient's age?
- A. age = int((birthdt-randdt)/365.25);
- B. age = int((randdt-birthdt)/365.25);
- C. age= int(yrdif(birthdt,randdt, "act/365.25" ));
- D. age = int((today()-birthdt)/365.25);
Answer:
B
Question 8
A Statistical Analysis Plan (SAP) defines the selection process for baseline records. This instructs the
programmer to choose the last non-missing analyte value prior to first study drug administration
(date/time).
The DEMO data set contains the date/time of first study drug administration for subject: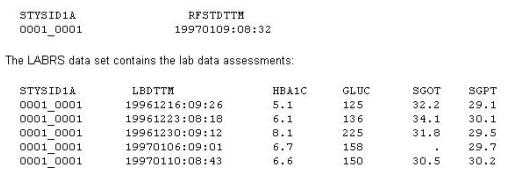
What will be the resulting baseline values, as selected per the SAP instructions?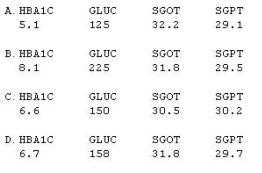
- A. Option A
- B. Option B
- C. Option C
- D. Option D
Answer:
D
Question 9
Which statement correctly describes an aspect of a Phase II clinical trial?
- A. randomized controlled multicenter trials on large patient groups
- B. designed to assess the pharmacovigilance, pharmacokinetics, and pharmacodynamics of a drug
- C. in vitro and in vivo experiments using wide-ranging doses of the drug
- D. designed to assess how well the drug works
Answer:
D
Question 10
CORRECT TEXT
Which CDISC filename contains the following items?
• Variable attributes
• Controlled terminology
• Computational methods
Enter your answer in the space below (Case is ignored. Do not add leading or trailing spaces to your
answer.).
Answer:
DEFINE.XML,DEFINE.PDF,DEFINE
Question 11
Where would you store a value collected on a case report form but not defined in an SDTM domain?
- A. RELREC
- B. DM
- C. SUPPQUAL
- D. SC
Answer:
C
Question 12
The purpose of the ADaM model is to provide a framework that:
- A. enables the tabulation of the raw source data
- B. enables the creation of study patient profiles
- C. enables the statistical analysis of the data
- D. can be used to generate the CDISC ODM
Answer:
C
Question 13
Given the following data set:
Which type of clinical trials data is this?
- A. Laboratory
- B. Baseline
- C. Medical History
- D. Vital Signs
Answer:
A
Question 14
Which CDISC standard is concerned with the development of simplified case report forms?
- A. Clinical Data Acquisition Standards Harmonization (CDASH)
- B. Operational Data Model (ODM)
- C. Study Data Tabulation Model (SDTM)
- D. Trial Design Model (TDM)
Answer:
A
Question 15
Identify the CDISC model with the following characteristics:
• XML-based content and format standard
• facilitates the archive and interchange of the metadata and data for clinical research
• provides an accurate audit trail that is 21 CRF Part II compliant
- A. Analysis Data Model (ADaM)
- B. Operational Data Model (ODM)
- C. Study Data Tabulation Model (SDTM)
- D. Trial Design Model (TDM)
Answer:
B